.jpg)
Figrue1. Electrophoresis for confirming recombination plasmid. lanes1: DNA Marker; lanes2: pET-28a(+)-((EK)10-TAT-LZE); lanes3: pRSFDuet-1-(LZR-ELP)-PAP.
And we expressed (EK)10-TAT-LZE with 0.4 mM IPTG induced for 5 h at 37℃ and did SDS-PAGE as well. The results showed that (EK)10-TAT-LZE was mainly expressed in supernatant, and it also performed a higher molecular mass due to the same reason mentioned above.
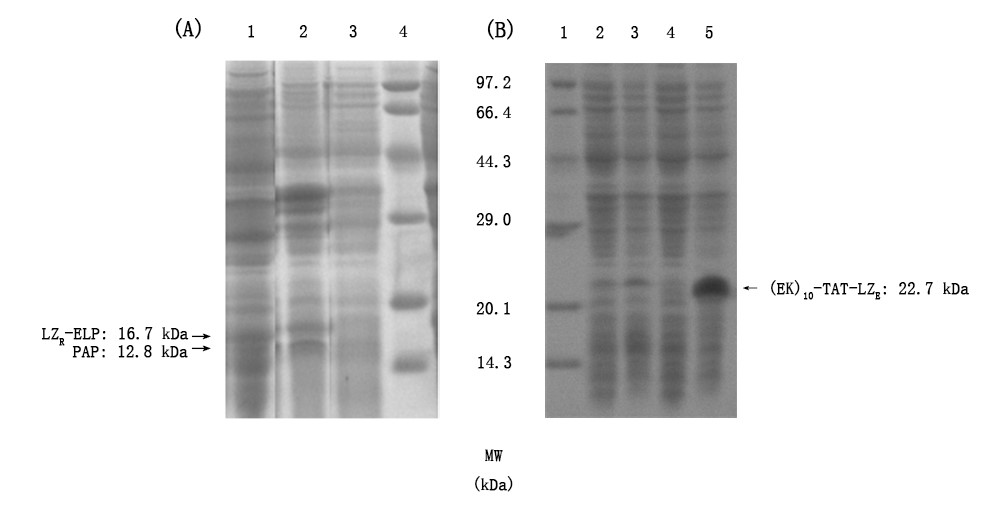
Figrue2. Induced expression and soluble analysis of LZR-ELP, PAP and (EK)10-TAT-LZE. a) determination expression form of LZR-ELP. Lanes1, supernatant and lanes2, precipitant obtained from induced bacteria; lanes3: bacteria liquid sample before induction; lanes4: Premixed Protein Marker (broad). b) determination expression form of (EK)10-TAT-LZE, lanes1: Premixed Protein Marker (broad); lanes2: precipitation obtained from induced bacteria; lanes3: bacteria liquid sample after induction; lanes4: bacteria liquid sample before induction and lanes5: supernatant obtained from induced bacterial.
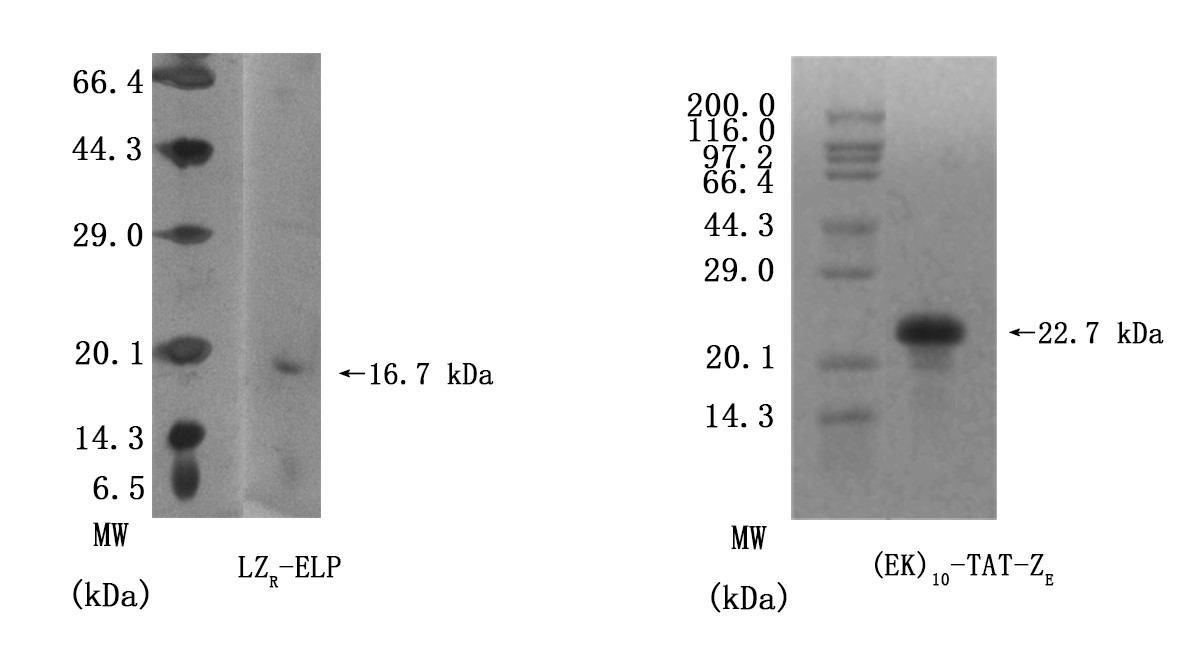
Figrue3. SDS-PAGE results of determining purities of LZR-ELP and (EK)10-TAT-LZE. Lanes1 and 2 were Premixed Protein Marker and target protein respectively.
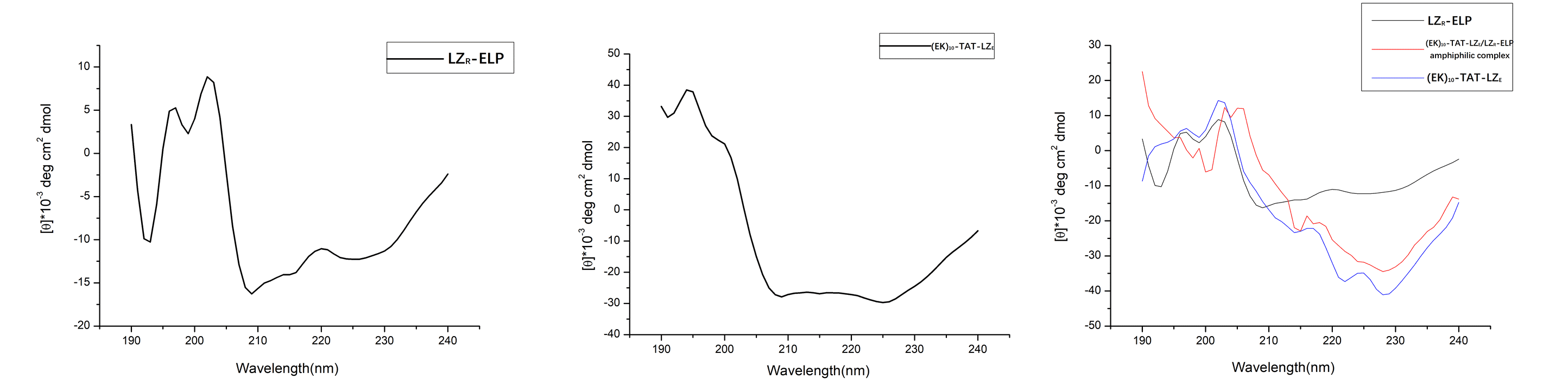
Figure4. (A) CD spectra of LZR-ELP (2×10-5 M). (B) CD spectra of (EK)10-TAT-LZE. (C) LZR-ELP (2×10-5 M) and (EK)10-TAT-LZE (2×10-5 M) assembled at pH=7.
However, due to the aggregation-induced light scattering of the leucine zipper [2], when mixed in equimolar amounts, the resulting spectrum showed less helical than the spectra for (EK)10-TAT-LZE (Fig. 4C). This indicated that the two building blocks interacted to form a (EK)10-TAT-LZE/LZR-ELP amphiphilic complex. This behavior demonstrated that our two building blocks can be dimerized by the leucine zipper with a secondary structure as the α-helix at 4℃, where hydrophobic of ELP is relatively weak to drive the assemble of forming the ERPCs.
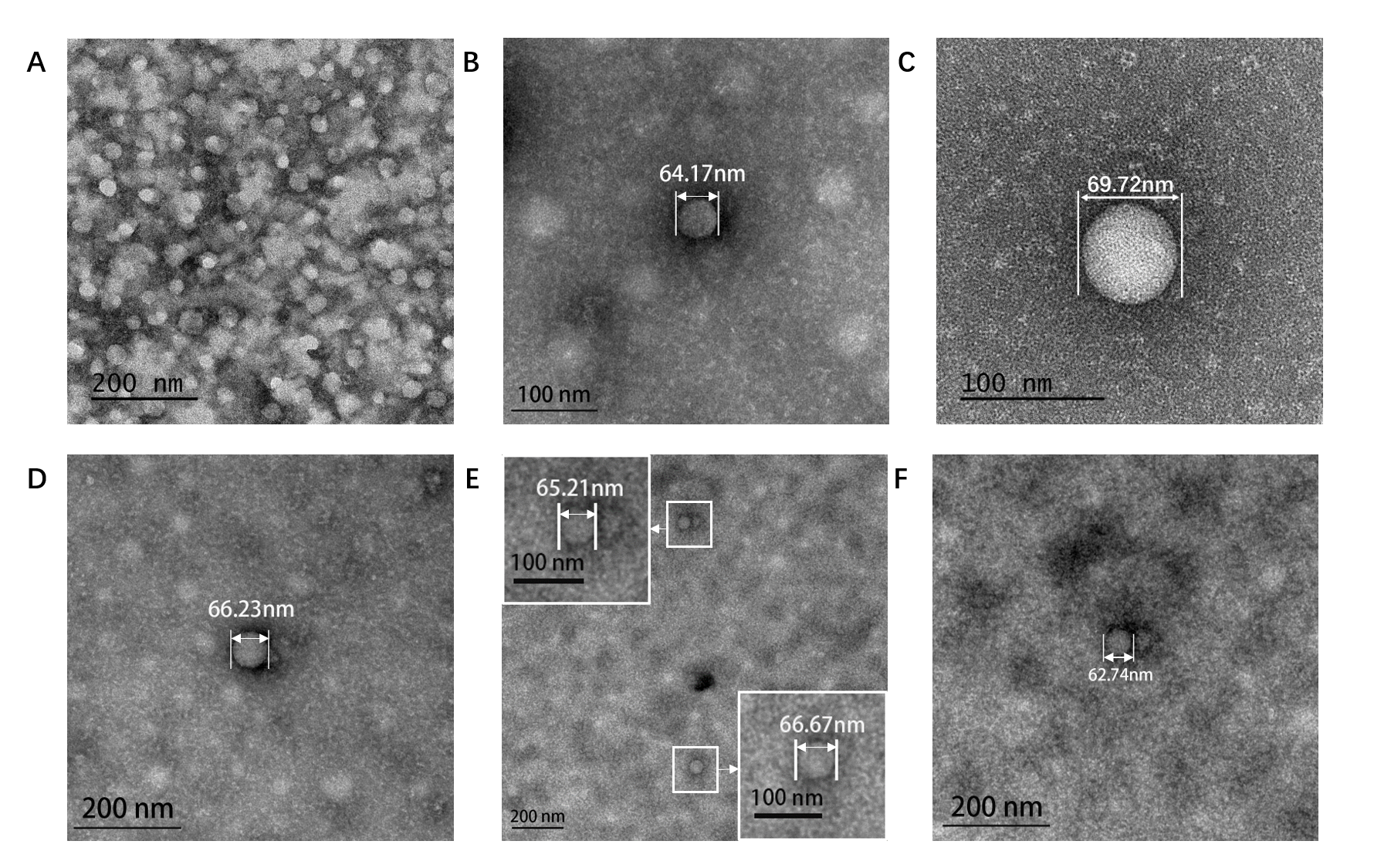
Figure5. TEM image of ERPCs which assembly at different conditions. (A). Assembly at 8.55×10-6 M (EK)10-TAT-LZE and 2.1×10-6 M LZR-ELP in pure water. (B). Assembly at 3.06×10-6 M (EK)10-TAT-LZE and 5×10-6 M LZR-ELP, 0.2 M PBS, pH=7.5, 0.175 M NaCl. (C). Assembly at 8.55×10-6 M (EK)10-TAT-LZE and 2.1×10-6 M LZR-ELP, 0.2 M PBS, pH=7.5, 0.3 M NaCl. (D). Assembly at 7.5×10-6 M LZR-ELP, 0.2 M PBS, pH=7.5, 0.6 M NaCl. (E)(F). Assembly at 8.55×10-6 M (EK)10-TAT-LZE and 2.1×10-6 M LZR-ELP, 0.9 M NaCl, 0.2 M PBS, pH=7.5. 0.9 M NaCl.
Salt concentration to be a critical factor for ELPs’ aggregation [2] and we tested the inverse phase transition of protein mixture solutions at different salt concentrations (0-0.9 M). The formation of ERPCs was only observed above the critical value of salt concentration, which is estimated to be approximately 0.175 M by TEM. Below this concentration, we only observed the formation of the protein random aggregation as the Fig. 5A. Above this concentration, ERPCs were present in the form of micelles, which had clear boundaries but not membrane structure in TEM image (Fig. 5 BCDEF). And the micelles under different salt concentrations exhibited uniform diameters as 60-80 nm.
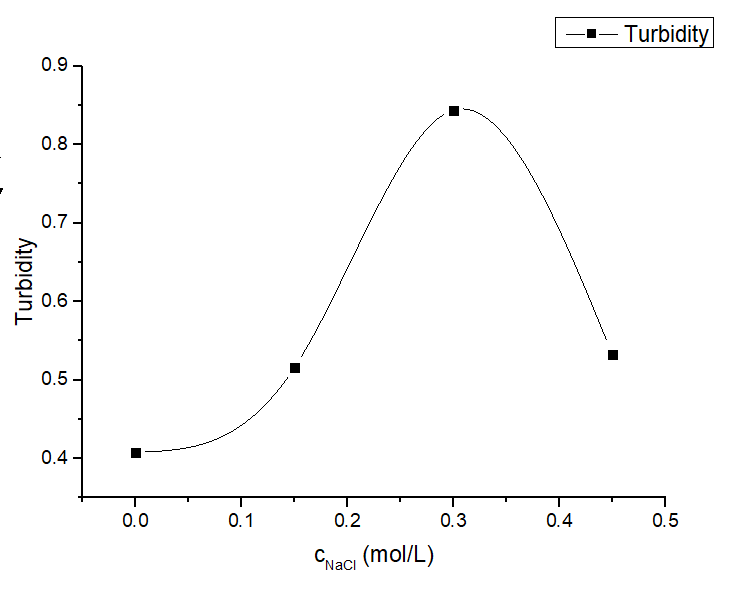
Figure 6. Turbidity profile of protein solution under various salt concentrations of protein mixture at different salt concentrations (0-0.45 M) with a fixed LZR-ELP and (EK)10-TAT-LZE concentration (12.5 μM and 3.125 μM).
Some of the assembled intermediate states were captured by TEM as shown in Fig. 7, which is of great significance to our study of ERPCs assembly. As can be seen from the Fig. 7, interestingly, the size of the core area of the ERPCs does not change significantly as the assembly process changes, which is consistent with the TEM image of Fig. 5B, D. We think its mechanism is ELP shows a β-helix structure while the two kinds of leucine zipper shows α-helix structure of LZR-ELP and (EK)10-TAT-LZE. Thus, the (EK)10-TAT-LZE/LZR-ELP amphiphilic complex structure had a high level of rigid which provided large amount of steric hindrance, as well as the charge carried by leucine zipper generated a complex electric field in space, making it difficult for the dimeric leucine zippers to spontaneously aggregate with others. Therefore, the particle size of the micelles is limited so that a uniform particle size was exhibited at different salt concentrations.

Figure5. TEM image of ERPCs procession. (A) is the formation of EPRCs (LZR-ELP 3×10-5 M, (EK)10-TAT-LZE: 2.4×10-5 M, with 0.1 M PBS and 0.6 M NaCl, assembling at 25℃) at earlier stage, (B) is the formation of ERPCs (LZR-ELP 2.21×10-5 M, (EK)10-TAT-LZE: 2.45×10-5 M, with 0.1 M PBS and 0.16 M NaCl, assembling at 37℃) at middle-late period and (C) has completed formation of ERPCs (LZR-ELP 2.21×10-5 M, (EK)10-TAT-LZE: 2.45×10-5 M, with 0.1 M PBS and 0.16 M NaCl, assembling at 37℃).
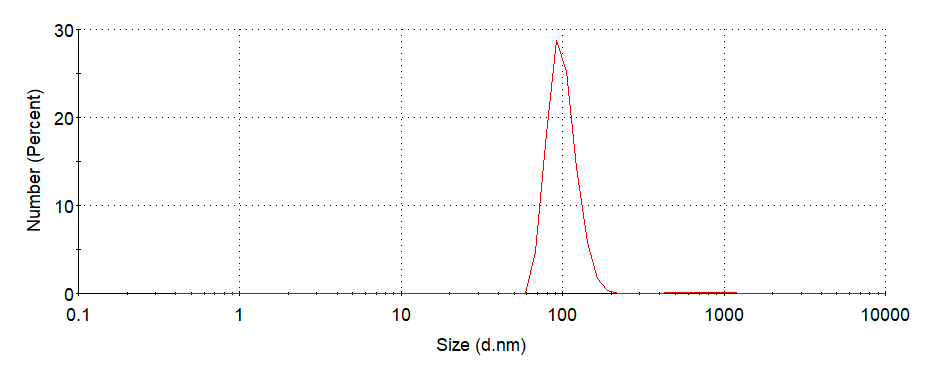
Figure6. Hydrodynamic radii(Rh)of ERPCs which assembled at 0.1 MPBS, 0.3 M NaCl, 37°C, pH=7.5. (PDI=0.302)
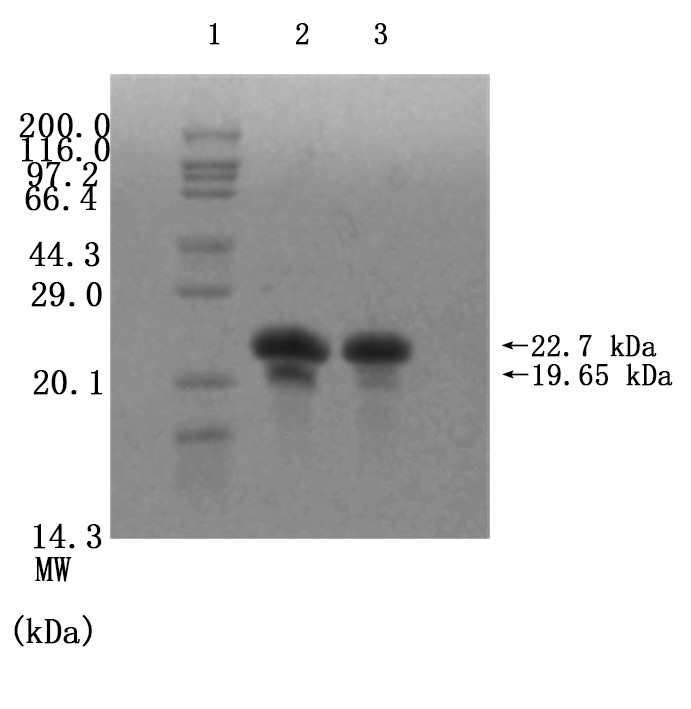
Figure7. Enzymatic cleavage. To determine the digestion of (EK)10-TAT-LZE(Left) and its nanopreparation (Right), the samples were treated with 1 μg/mL MMP2 followed by SDS-PAGE. Assembly at 0.1 M PBS, 0.3 M NaCl, 37°C, pH=7.5
We mixed 3.12×10-6 M (EK)10-TAT-LZE and 1 μg/mL human MMP2 then incubated at 37℃ overnight, to let the MMP2 bind to its sites on functional block. And then we did SDS-PAGE to examine if the enzyme sites effective. Compared with Fig7. Right, one new band was seen in the gel image of Fig. 7 Left. The results indicated that the MMP2 cleaved the (EK)10-TAT-LZE, resulting in two digestion fragments even though one’s molecular weights was too small (about 3.05 kD) to obtain a longer residence time in the gel. Furthermore, we characterize the zeta potential by DLS to demonstrate the surface properties of pre- and post-enzyme digestion. Incubation of ERPCs with MMP2 showed charge change (Fig. 8), this strongly suggested that the sites of our carrier are accessible even in the “compact” micellar structure[4]. The result of zeta potential was shown in Fig. 8. ERPCs obtained a anionic surface before the digestion of MMP2 due to the (EK)10 block, and after the digestion of MMP2, the exposition of TAT peptide endows the ERPCs cationic surface (Fig. 8B).
This result revealed that the environmentally responsive property of ERPCs through over-expressed MMP2 enzyme digestion is feasible, while the electrification effect responses to environment change presents advantages of transport and cell internalization [5]. We are going to conduct cell experiments for further study.
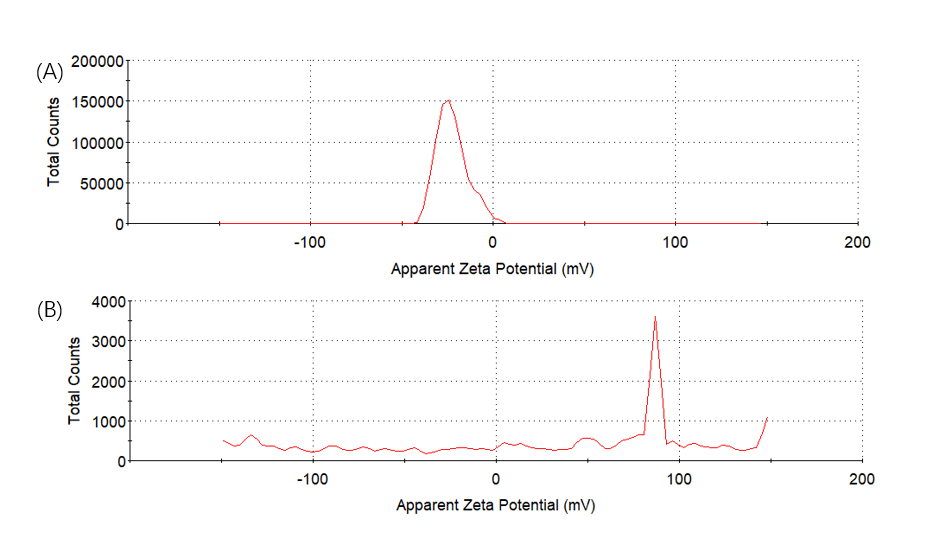
Figure8. Zeta potential. To determine the charge change of digestion of (EK)10-TAT-LZE (A) and its nanopreparation (B) the samples were treated with 1 μg/mL MMP2 followed by SDS-PAGE.
Based on these results, we hope our methods can contributed to the diversity of nucleic and hydrophobic drugs and give some inspiration to the field of controllable nano-synthesis. Theoretical calculation has revealed that LZR-ELP can assembled by itself. This means the design of another building block has been given great diversity and more complicated functions can be realized. In the future, assemblies constructed using this strategy may also be useful in the fields of catalysis, protein engineering, nano-robot, bionanotechnology and synthetic biology, which are far beyond the usage in drug delivery.