DESIGN
We designed a covalently bonded vesicle that can be constructed by substrate-free self-assembly at room temperature. Biotin and i-motif were added to this vesicle so that it can be readily functionalized with streptavidin while its pore size responds to pH changes.



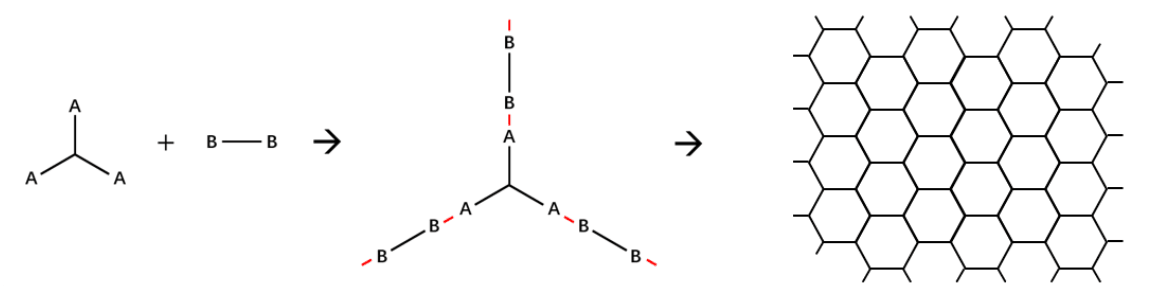
Figure 1. Common mechanism to construct two-dimensional nanostructures.
Building blocks possessing multiple in-plane reactive groups at the periphery (An) and difunctional linkers (B2) are typically used to construct nanostructures (Figure 1). Therefore, the core of our design is to select a rational interaction between the building blocks and the linkers. We chose a reversible reaction, aldimine condensation, to connect our building blocks and linkers in order to assemble the vesicle (Figure 2). The imine linkages formed by the condensation have four characteristics, which are particularly favorable to our assembly.
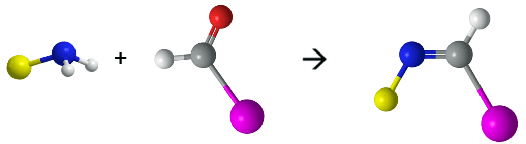
Figure 2. Ball-and-stick model of aldimine condensation.
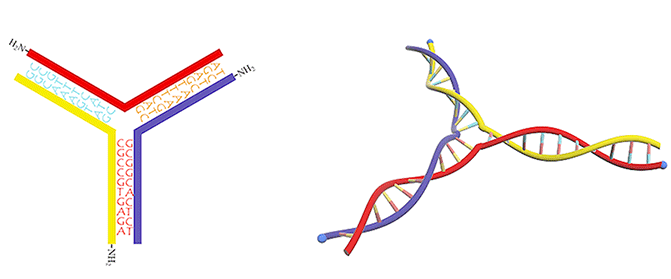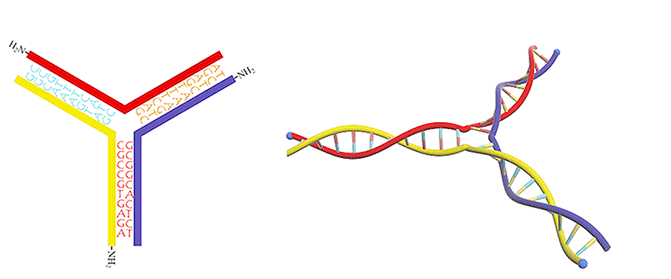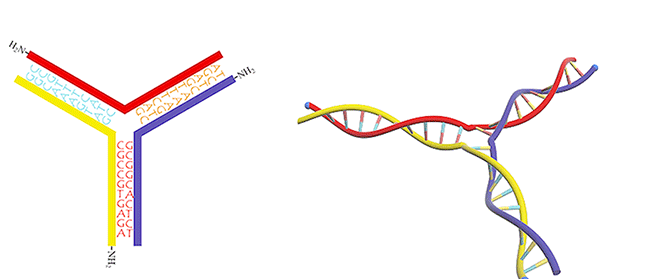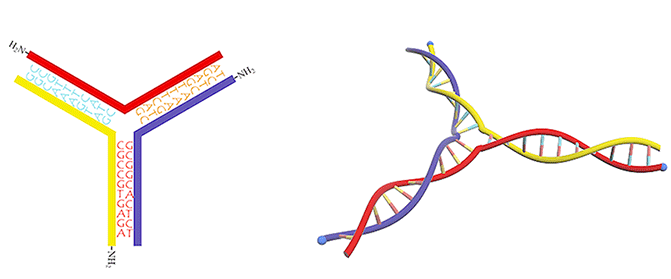
Figure 3. DNA three-way junction modified with amino groups.
![]() Unlike the noncovalent interaction in a supramolecular system, our linkage is a covalent bond, which allows for the stability of the vesicle.
Unlike the noncovalent interaction in a supramolecular system, our linkage is a covalent bond, which allows for the stability of the vesicle.
![]() The imine linkages can be formed at room temperature, without perturbation to some active molecules or enzymes.
The imine linkages can be formed at room temperature, without perturbation to some active molecules or enzymes.
![]() Since aldimine condensation is a reversible reaction, the imine linkage can break down and restructure itself, ultimately leading to the formation of the thermodynamically most stable species.
Since aldimine condensation is a reversible reaction, the imine linkage can break down and restructure itself, ultimately leading to the formation of the thermodynamically most stable species.
![]() The imine linkages can be easily reduced to C-N bond after assembly. Since C-N bond is much more stable than C=N bond, the reduction would undoubtedly further enhance the stability of the vesicle.
The imine linkages can be easily reduced to C-N bond after assembly. Since C-N bond is much more stable than C=N bond, the reduction would undoubtedly further enhance the stability of the vesicle.
We utilized a DNA three-way junction as our building block, which is composed of three single-stranded DNAs (ssDNAs) partly complementary to each other, with amino groups modified at their 5-prime ends (Figure 3).
In this case, dialdehyde is applied as a linker to connect DNA three-way junctions. The aldimine condensation between amino groups and aldehyde groups results in the formation of a two-dimensional DNA nanostructure, a grid with polygonal pores (Figure 4), the intermediate of vesicle assembly (Figure 5). Furthermore, we modified biotin (Figure 6) at the 3-prime ends of ssDNAs (Figure 7), enabling the vesicle to be functionalized by streptavidin.
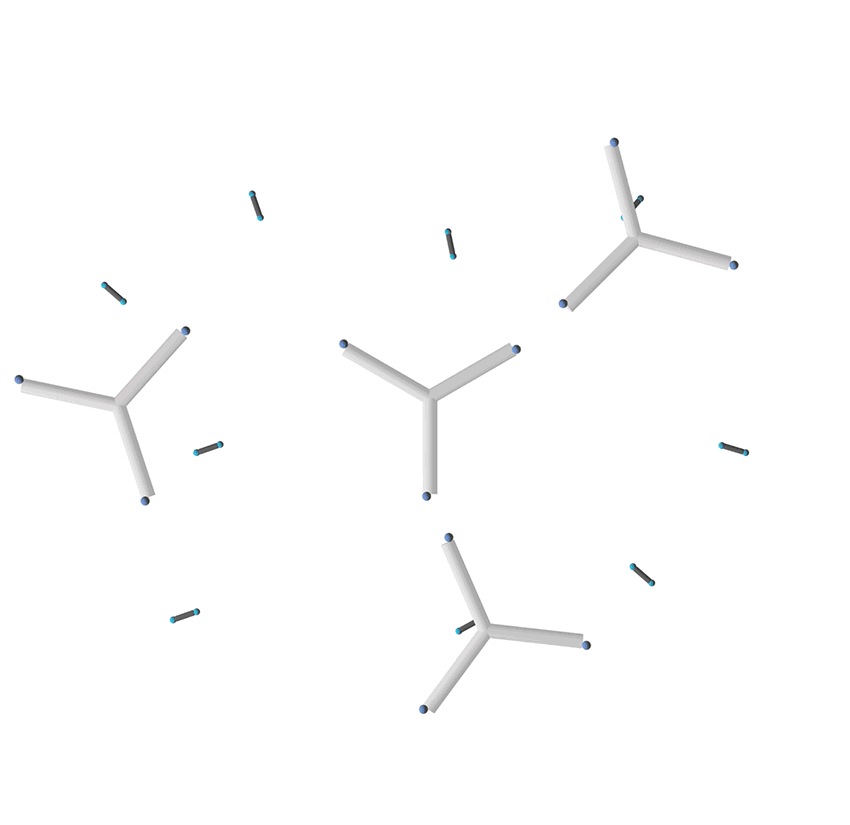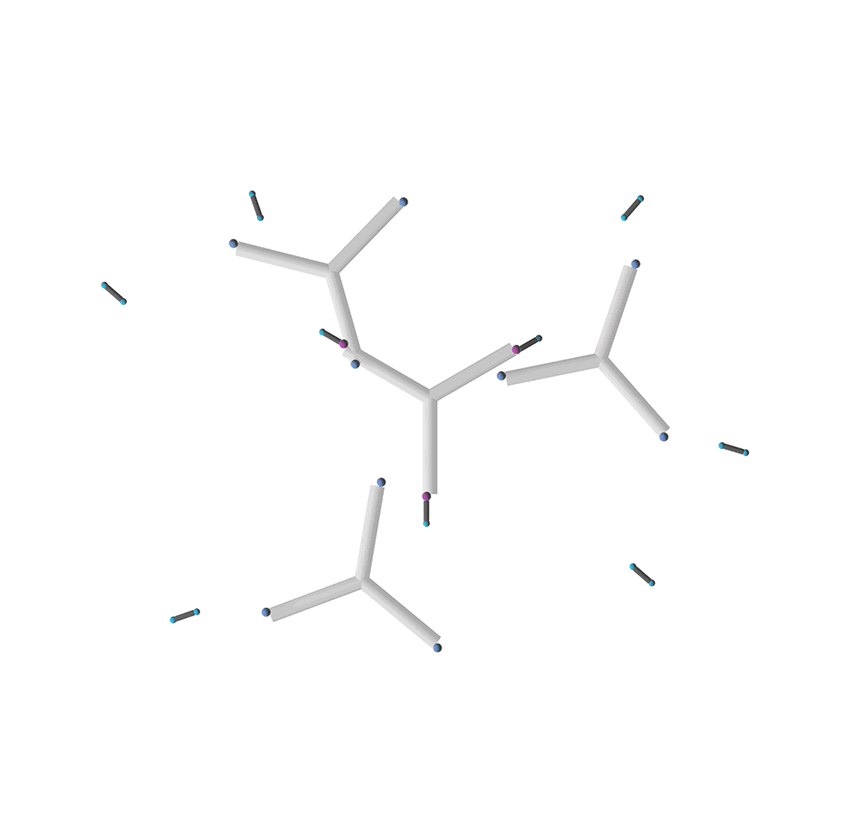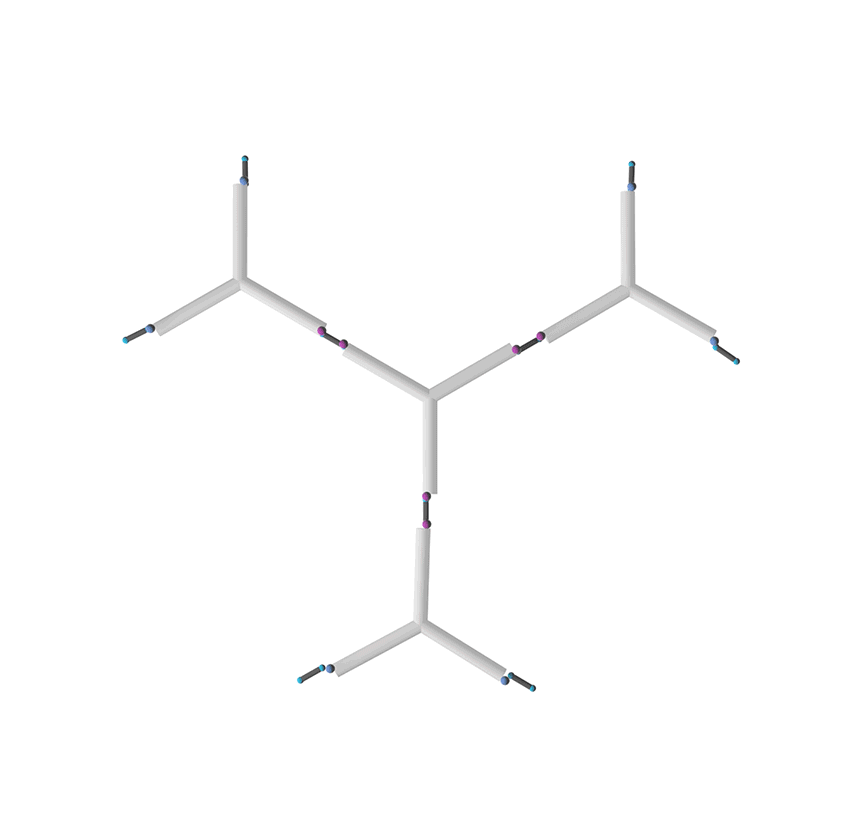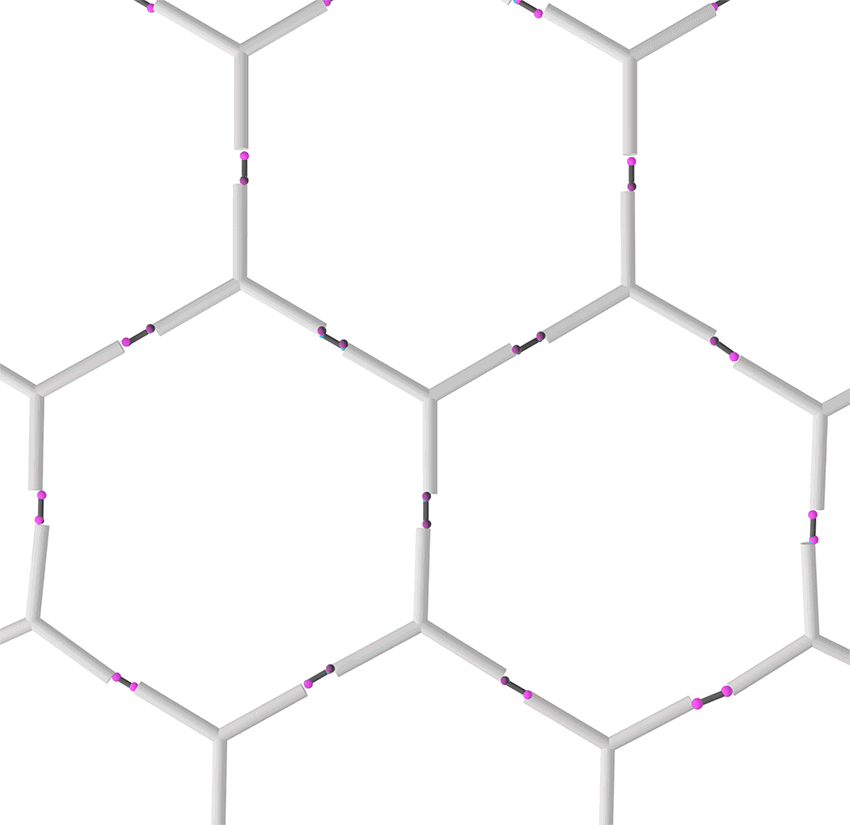
Figure 4. Formation of a two dimensional DNA nanostructure.

Figure 5. Formation of a DNA vesicle.
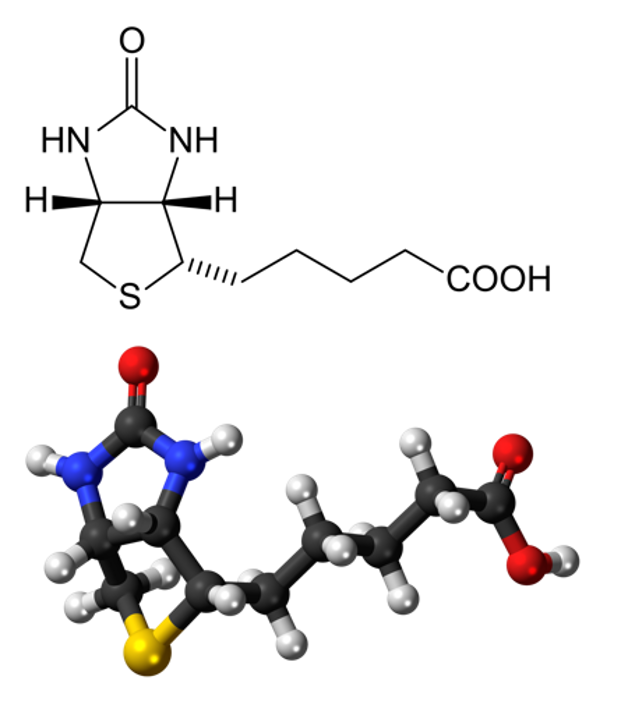
Figure 6. Biotin
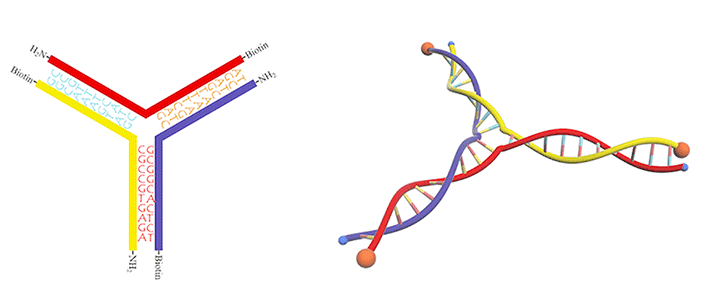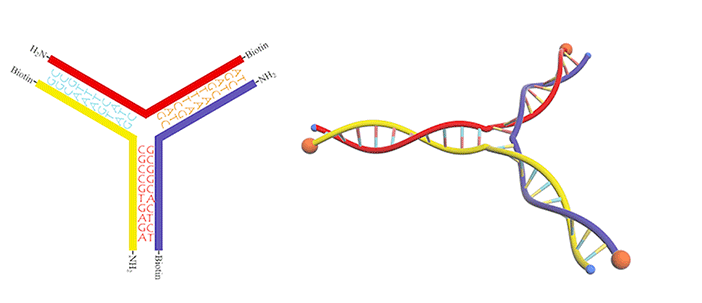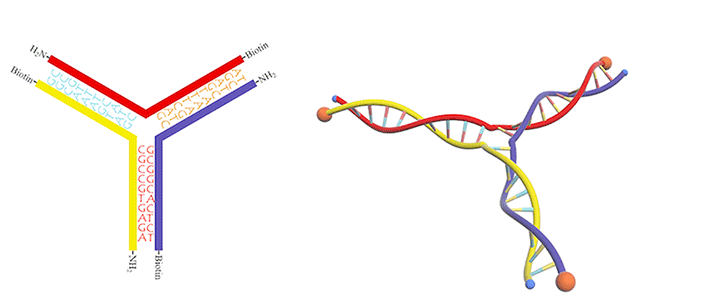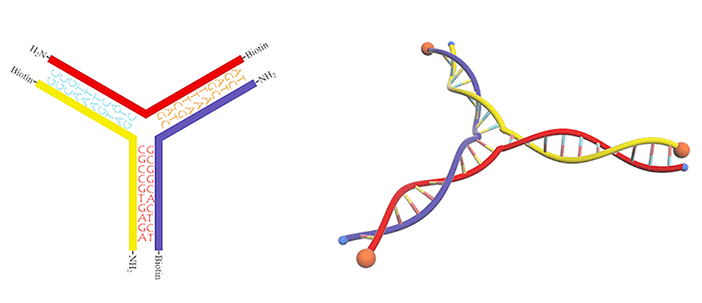
Figure 7.DNA three-way junction modified with amino groups and biotins.
Besides, using DNA as building material enables the effortless introduction of the i-motif structure (Figure 8).
We inserted the i-motif sequence between the complementary parts and amino groups (Figure 9), so that when pH value becomes higher, the i-motif disassemble; thus, increasing the pore size of the vesicle (Figure 10).
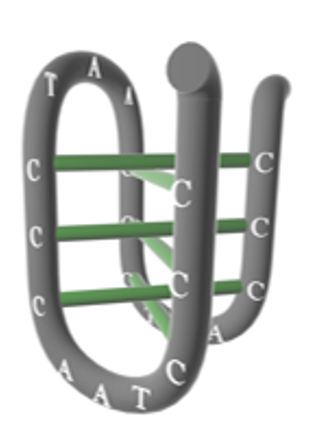
Figure 8. i-motif
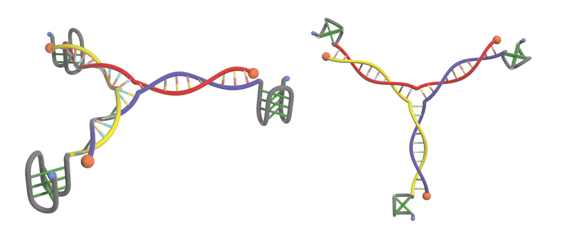
Figure 9. Final building block:DNA three-way junction involving biotin and i-motif structure.
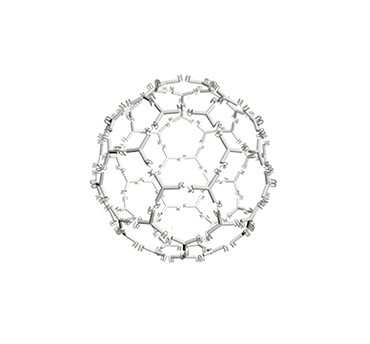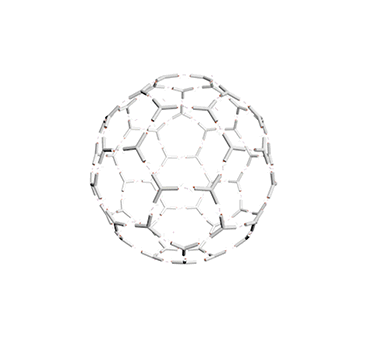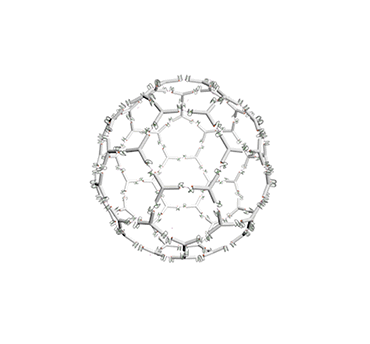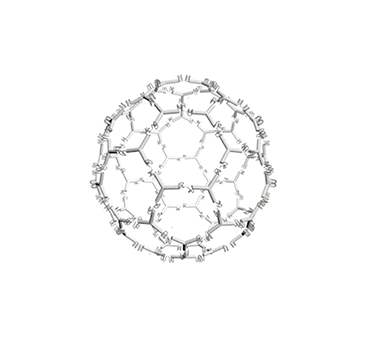
Figure 10. The vesicle responding to pH changes.
The vesicle might serve as a prototype of drug carrier, which has the potential to realize targeted drug delivery and pH-controlled release through the following three steps.
1. Drug molecules are trapped in the vesicle during co-assembly at room temperature.
2. Through biotin-avidin interaction, the vesicle binds to a functional fusion protein, which recognizes our target.
3. Drug molecules are released to the target, through the enlarged pores from the change in pH value.