











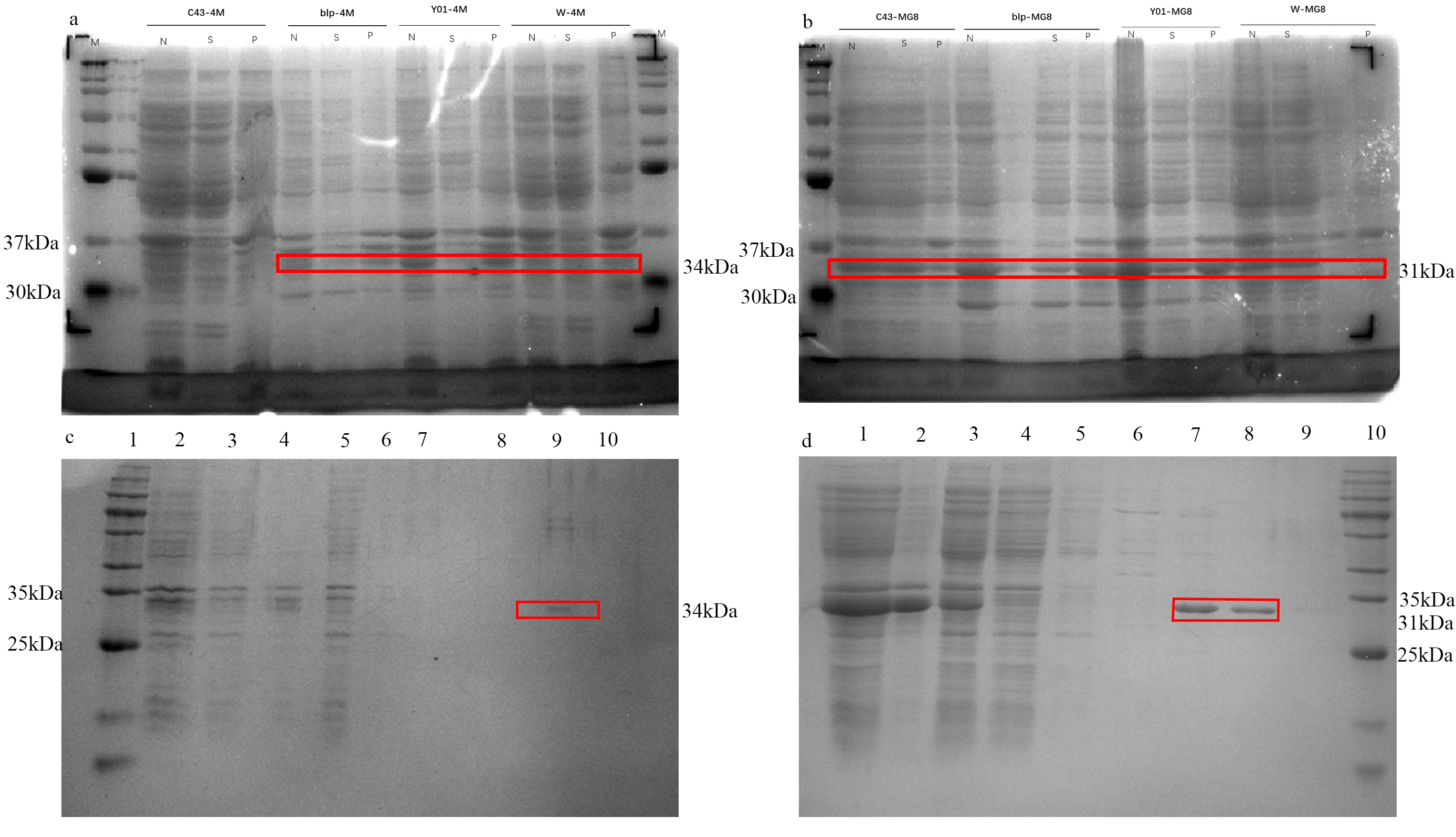
The expression levels of target protein genes on the plasmids varies in different bacteria. It is significant to keep both of 4M and MG8 substantial expression. MG8-pET28a and 4M-pET28a were transformed into Y01, BLP, C43, W competent, incubated according to method 2 to select the best expressing bacteria. Fig.1a and 1b show that 4M had a higher expression level in BLP and that of MG8 were higher in Y01. The molecular weight was the same as prediction (MG8 is 34kDa, 4M is 31kDa). The purification experiments were done with the selected bacteria.
To keep our system free from excessive interference from other hetero-proteins, we purified the crude enzymes. Two enzymes with His+ tag were subjected to affinity chromatography using a Ni2+-NTA column, and finally were eluted at 100mM imidazole. The SDS-PAGE showed that the purified proteins were available for subsequent studies of catalytic properties of the 4M and MG8.
4M and MG8 surface contain several kinds of primary amine groups: α-amine group at the N-terminus and ε-amino groups at the lysine residue. They were immobilized on the Fe3O4 NPs with carboxyl groups which can form covalent bond respectively by using the method 4. After the immobilization on Fe3O4 NPs (1138.67nm), the average diameter of MG8@Fe3O4 (1182.2nm) and 4M@Fe3O4 (1260.9nm) remained almost constant (Fig.2a). The results showed that immobilization had little effect on the dispersion of Fe3O4 NPs. Furthermore, to test whether they were immobilized, we measured their ζ-potential. ζ-potential of bare Fe3O4 NPs is negative (-48.9±2.7 mV) due to the rich citrate ions on the surface. The strong electrostatic repulsion between Fe3O4 NPs results in good dispersion in solution. The ζ-potential of 4M@Fe3O4 (-15.9±0.53 mV) and MG8@Fe3O4 (-16.5±0.4mV, Fig.2b) were lower than that of bare Fe3O4 NPs. This may be due to the introduction of proteins with low ζ-potential, which obscured the surface charge of Fe3O4 NPs. Both experiments above indicated that the enzymes were immobilized successfully.
Afterwards, magnet was used to separate the MG8@Fe3O4 or 4M@Fe3O4 from solution. The remaining enzymes concentration were quantified by using BCA protein assay kit. The concentration of 4M and MG8 were 0.016mg/L and 0.034mg/L. Fig.2c demonstrated the immobilization efficiency of 4M and MG8 were 83% and 65%. MG8 had a lower immobilization efficiency may be due to the lower number of amino groups on its surface. To explore if Fe3O4 NPs could degrade p-nitrophenol acetate (pNPA), hydrolysis product of Fe3O4 NPs reacted with pNPA was determined, which showed that there was little p-nitrophenol (p-NP) generated. It was proved that Fe3O4 NPs did not cause the hydrolysis of pNPA, having little influence on the reaction.

4M is a kind of serine hydrolase and MG8 has esterase activity, which possesses the ability to hydrolyze p-NPA into p-NP. As reported in literature, p-NPA has been widely used as a model substrate to assess the enzymatic properties of PETase. Here, enzyme activity was measured by using pNPA. We used microplate reader to test the concentration of p-NP after reacting 2min under the condition of 55˚C(4M) and 40˚C(MG8). If the enzymatic reaction is carried out at a sufficiently large substrate concentration without inhibition, the reaction rate is proportional to the concentration of the enzyme, closing to the initial reaction rate. We define the unit of activity of an enzyme as the amount of enzyme consumed to produce 1 μmol of p-NP in 1 min. When the amount of 4M enzyme is 0.036g and MG8 is 1.6g, the enzyme activity is 1U. the activity of 4M was higher than that of MG8 at the optimum temperature respectively. Table.1 is the enzyme activity for per gram protein.
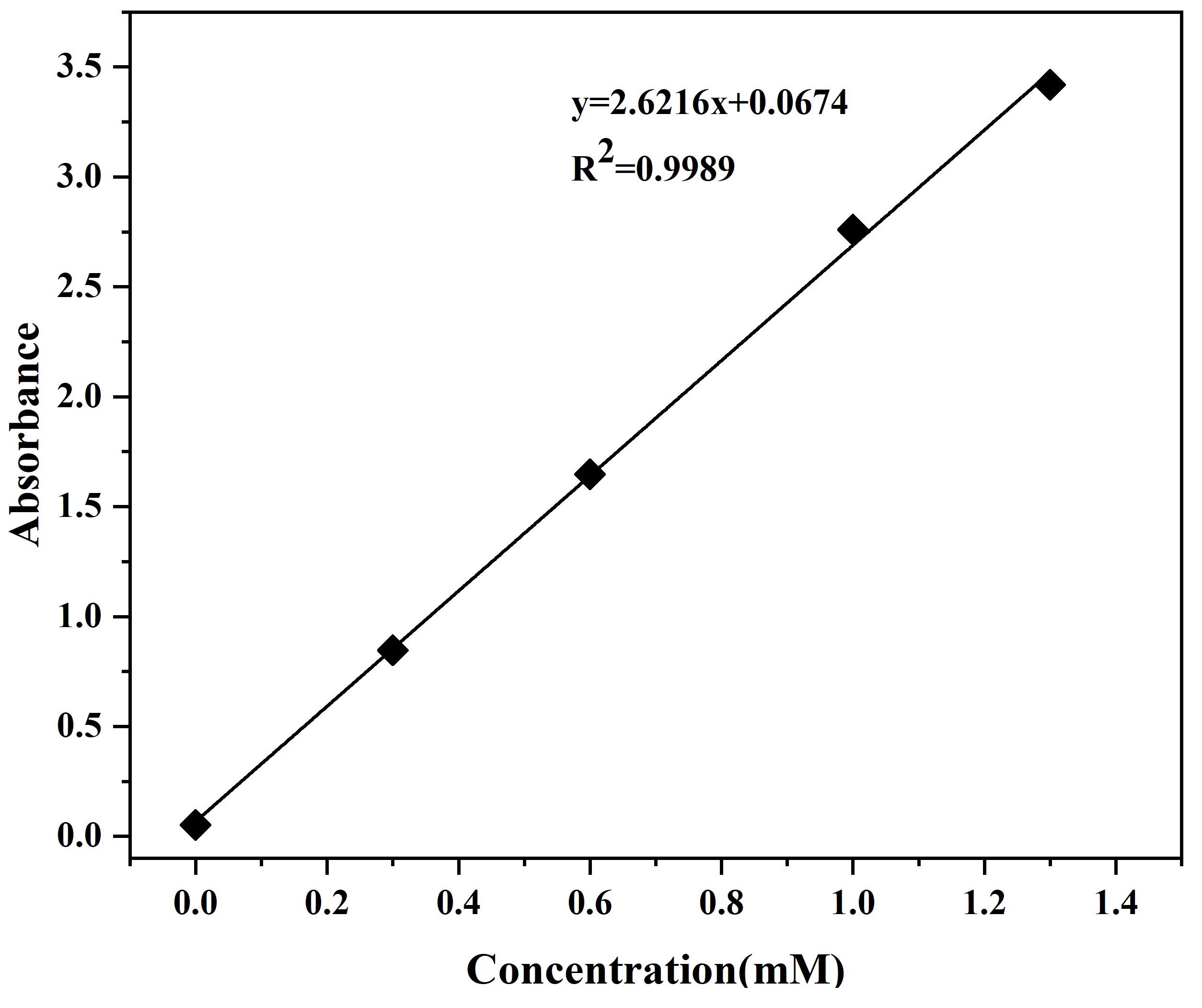
To quantify the concentration of p-NP a product of pNPA decomposition, we prepared the solutions with a concentration gradient using p-NP and tested its absorbance change at 405nm with the change of concentration. The data were fitted to a linear function: y = 2.6216x + 0.0674 (R² = 0.9989), which indicates a good result of fitting.

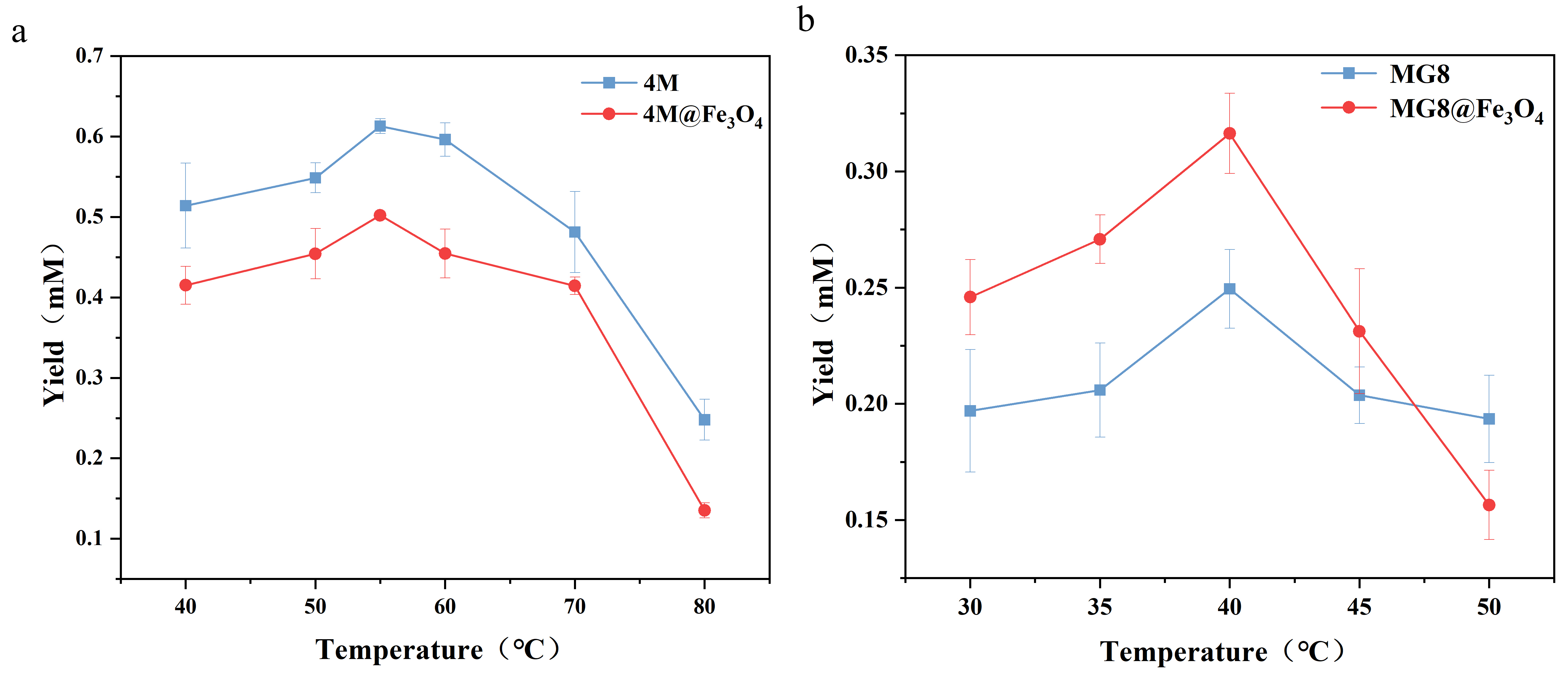
The reaction catalyzed by enzymes is often limited by factors such as decreased enzyme activity. And temperature as the main factor affecting enzyme activity, which affects the rate of enzyme-catalyzed reactions in two ways. Firstly, the increase of temperature enhances the thermal energy of the substrate molecule to accelerate the reaction. Secondly, high temperature leads to the disruption of multiple non-covalent bonds, which maintain the tertiary structure of the enzyme and the disruption of these bonds. Eventually, it will lead to the denaturation of enzymes, reducing the enzyme activity.
The effects of temperature on the properties of free and immobilized enzymes were determined using pNPA as substrate. We found that the activity of 4M was higher between 40˚C and 70˚C. Its optimum temperature was 55˚C. And, the activity of MG8 rose between 30°C and 40°C and its optimum temperature was 40°C. It was showed from the Fig.4 that immobilization did not seem to change the optimum temperature of the enzyme significantly, owing to the fact that the stability of the free enzymes with a disulfide bond to form rigid structure were superior.
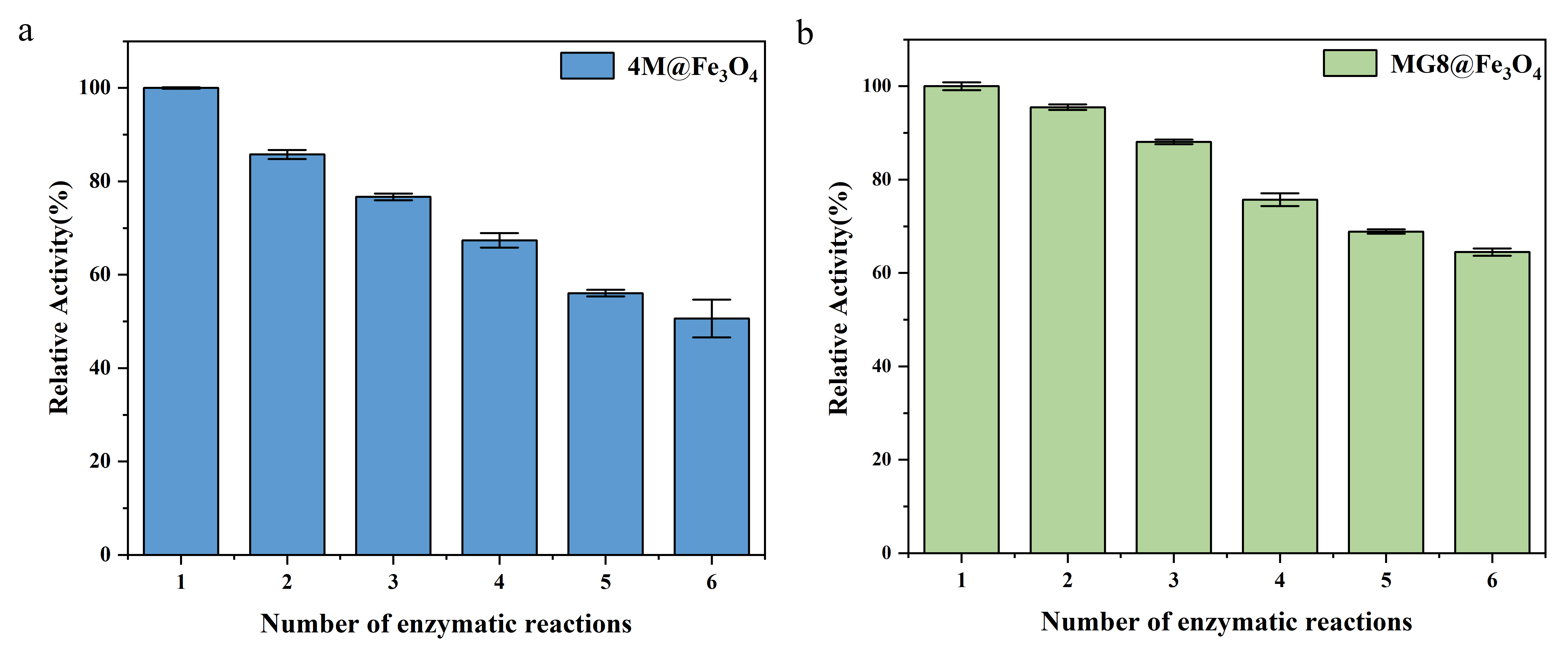
The immobilization could not only improve the adaptability of the enzyme to the environment, but also increase recycling efficiency. For the general reaction, it was tough to recycle the enzyme in solution, and it was a waste. However, in this experiment, the enzyme and product in solution were separated after immobilizing, according to the method 5. The product p-NP was detected by ultraviolet spectrophotometer. After recycling 4M@Fe3O4 and MG8@Fe3O4 for 5 times and re-reaction, it was found that residual activity of 4M@Fe3O4 still maintained 50.64%, and that of MG8@Fe3O4 kept up to 64.48% after being recycled six times, which indicated that they had potential reusability. It was reported that enzyme activity decreased in general experiment. The reasons why the loss of enzyme activity might be as follow: the immobilized enzyme could not be completely recycled and might be lost in the process of reaction. Under these circumstances, repeated recycled and dissolution of the enzyme might result in inactivation of the enzyme[1].
According to our design, 4M and MG8 can degrade different form PET plastics. After co-immobilizing the two enzymes on the same carrier, we can get a complex(4M-MG8@Fe3O4) which has the ability to catalyze different forms of polymerization PET simultaneously, thus achieving different functions.
According to the optimum activity ratio determined on the basis of free enzyme 4M, MG8 were added at 1U and 1U, but we can’t ignore the effect of protein loss due to immobilization. The immobilization efficiency of enzymes is 83% (4M) and 65% (MG8). If the enzyme activity was xU, theoretically immobilization efficiency of the immobilized enzyme would be 100%.the corresponding relationship was 1U × 100%=xU × 83%, and the actual dosage was x = 1U × 100%/ 83%=1.2U. Using the same method, the actual dosage of MG8 was 1.54U. So we can know the ratio of free enzyme is 1.20 U: 1.54 U.